If you need CDISC services for clinical trials, please contact us at info@sofpromed.com
Clinical trial data standardization is important when it comes to data management and clinical data submission to regulatory authorities. Having datasets structured in a consistent manner facilitates the processes of data collection, analysis, and exchange.
- $What Is CDISC?
- $What Are the Main CDISC Standards?
- $What Are the Main Advantages of Using CDISC Standards?
- $Is CDISC Required by the FDA?
- $Is CDISC Required by Other Regulatory Agencies Worldwide?
- $How Can a Clinical Trial Sponsor Implement CDISC Standards in its Clinical Trial?
- $What CDISC Services Does Sofpromed Provide?
Since its inception in 1997, CDISC has been concerned with the need for the establishment and development of international industry standards with the aim of supporting the acquisition, exchange, submission, and classification of clinical trials information for biopharmaceutical product development.
Throughout this article, we will offer an overview of the main CDISC standards and discuss whether the FDA requires CDISC for data submissions.
What Is CDISC?
CDISC, which stands for the Clinical Data Interchange Standards Consortium, is a global standards developing organization (SDO) dealing with clinical research data. Even though CDISC emerged as a volunteer group in 1997, it would become an independent, non-profit organization in February 2000.
The main objective of the consortium is to develop data standards for the collection, analysis, and transmission of clinical data. Not only that, but it also promotes the use of these standards to enhance the quality and interoperability of medical research. CDISC is mostly used for clinical data management and analysis by biotech and pharmaceutical companies as well as for data submissions to regulatory agencies worldwide.
Certainly, the development of clinical data standards would not be achieved without the collaboration and assistance of world leading health care professionals who provide their vision, knowledge, and expertise. In order to ensure that they actually reflect the current needs and practices of the scientific research community, this process is performed on the basis of a general consensus of all participants.
The consortium works in cooperation with world recognized global agencies whose influence in decision-making and CDISC data standards development can be crucial. Some of them include the U.S. Food and Drug Administration (FDA), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), the European Medicines Agency (EMA), and China’s National Medical Products Administration (NMPA).
In addition, CDISC’s membership is also formed by a great variety of groups relating to medicine and clinical research. These contributors are, for instance, clinical research organizations, pharmaceutical and biotech companies, technology service and healthcare providers, among others. All stakeholders are bonded together by a common interest: to maximize the impact of data on clinical research. [1]
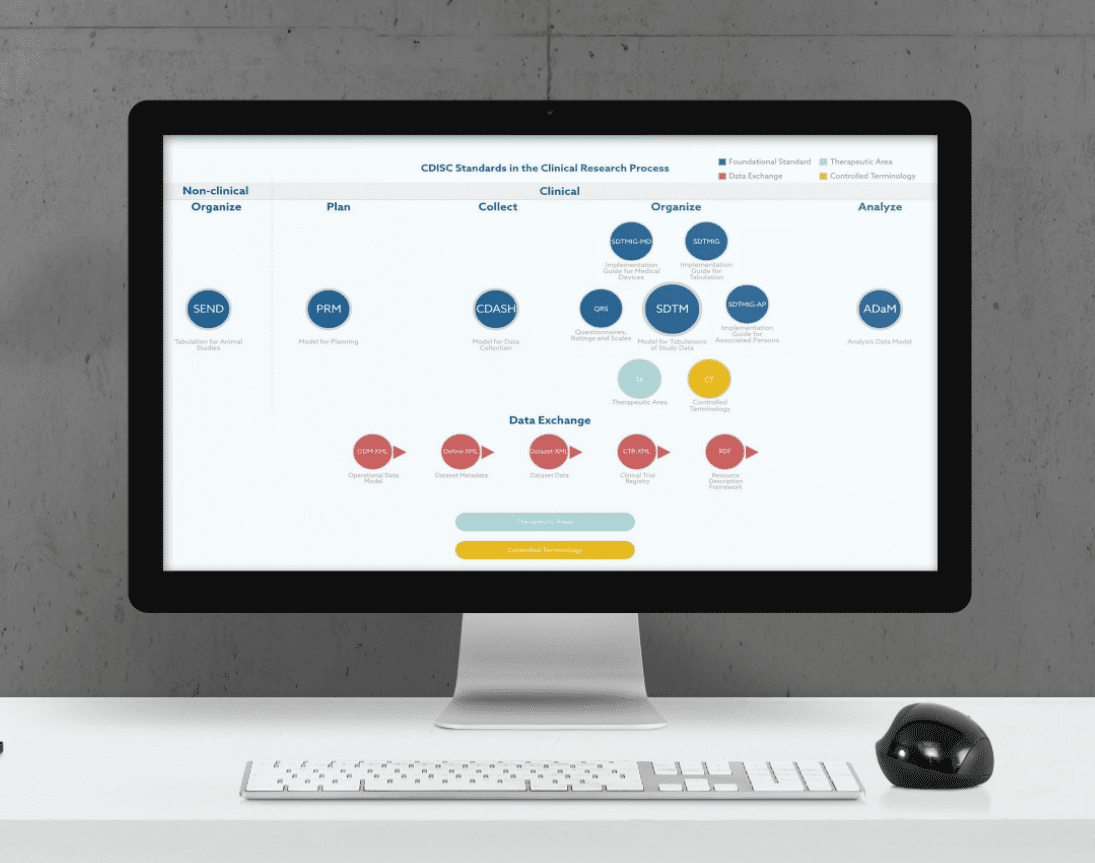
What Are the Main CDISC Standards?
CDISC standards have proven to be very helpful as they make clinical data clearer and, therefore, easier to interpret. Moreover, they have been shown to reduce the number of resources needed by 70-90% when they are applied in the early stages of a clinical research study.
As a result, the implementation of CDISC standards has become an integral part of the data management processes taking place at all phases of a drug development program.
Although the importance of SEND as a CDISC standard for non-clinical studies should not be underestimated, in this article we will focus on the three major CDISC standards for clinical research studies, which are CDASH, SDTM, and ADaM. A brief explanation of the main features and objectives of each CDISC standard is presented below.
CDASH
CDASH stands for Clinical Data Acquisition Standards Harmonization. This set of standards allows clinical data to be collected in a precise and consistent way across studies and sponsors.
More specifically, CDASH provides guidance to develop the case report form (CRF), which is designed by the clinical data manager with the help of the statistician, the medical monitor, the sponsor, and the clinical operations team. The goal of CDASH is to describe best practices to develop CRFs.
Furthermore, CDASH classifies data collection fields in three different categories, depending on their grade of recommendation: [2]
- Highly recommended (HR): This data collection field should be on the CRF.
- Recommended/conditional (R/C): This data collection field should be on the CRF under certain conditions.
- Optional (O): This data collection field is appropriate or available for use.
In this sense, CDASH’s approach has proven to be so efficient and systematic that many relevant regulatory authorities, such as the FDA, have recommended it for clinical data collection.
SDTM
SDTM is an acronym for Study Data Tabulation Model. It defines a standard used for organizing data collected in human clinical trials and nonclinical studies. In particular, SDTM organizes and formats data tabulations before their submission to a regulatory authority in the process of a product application.
SDTM provides an accurate description of the structure, contents, and attributes of each dataset, as well as the variables submitted as part of the clinical research study. Before its implementation, the existence of different variables, domain names and structures resulted in more time and effort being necessary for reviewers to get the data into a standard format.
Nonetheless, thanks to SDTM, now there are standard domain names, standard structures for each domain, standard variables, and standard names for SDTM datasets. As a result, data can be more easily identified. [3]
Definitely, SDTM is essential to accelerate and enhance regulatory review and approval processes. This is evidenced by the fact that SDTM is the required standard for data submission to the US FDA and Japan’s PMDA.
ADaM
The Analysis Data Model (ADaM) specifies basic principles and standards for statistical and scientific analysis. Once SDTM datasets for organizing clinical trial data have been provided, ADaM offers a connection between these SDTM datasets and the final statistical analyses.
ADaM makes sure that data are adequately transferred, reproduced, and traced by doing the following: defining main principles for the creation of analysis datasets, establishing steps for dataset creation and result generation, describing different types of metadata, and specifying dataset naming, content, and order of variables. [4]
Hence, due to its wide variety of functions, ADaM has become one of the most required standards for clinical data submission.
What Are the Main Advantages of Using CDISC Standards?
Actually, there are many reasons why CDISC standards should be regarded as an opportunity for biotech and pharmaceutical companies. According to the CDISC official website, these are some of the benefits of implementing CDISC standards: [5]
- Fostered efficiency
- Complete traceability
- Enhanced innovation
- Improved data quality
- Facilitated data sharing
- Reduced costs
- Increased predictability
- Streamlined processes
In addition to the advantages mentioned above, CDISC standards support transparency and data understandability throughout the whole clinical trial. Not only that, but the implementation of CDISC standards also means a significant decrease of costs and timelines during product development, as well as a reduction of the amount of time that regulatory authorities need for data review and approval processes.
The standardization of data also implies reduced training costs and requirements. The reason behind that is that new workers in the Pharmaceutical industry will not be required to learn company-specific data standards in each case. The same applies to CROs: they will not have to use different standards for every client. Instead, they can simply adopt universal CDISC standards.
On that account, the use of CDISC standards enables the interpretability of data, which thus can be transferred and exchanged in an easy and efficient manner.
Is CDISC Required by the FDA?
The US Food and Drug Administration (FDA) is a federal agency within the Department of Health and Human Services. Since its very beginning in the early twentieth century, FDA’s mission has been to protect and promote public health.
Moreover, FDA tackles the challenges posed by the constantly evolving laws governing the industries that it regulates, supports strategic priorities, and boosts the role of health offices, teams, and centers. The ultimate aim of this consumer protection agency is to guarantee that medical products are safe and effective, and help the public obtain accurate information before starting any new drug treatment.
Apropos the issue at hand, the short answer is ‘yes’. The FDA is clearly a senior member of CDISC standards, and these are required for regulatory submissions to FDA. As a matter of fact, the two organizations have always worked together very closely to ensure that CDISC data standards succeed in allowing regulatory reviewers to receive, process, revise, and store submissions in a systematic way.
Is CDISC Required by Other Regulatory Agencies Worldwide?
CDISC data standards are not solely required by the FDA. In actuality, other regulatory agencies have also shown their commitment to modernize and streamline the review process by employing innovative analysis tools, using them more consistently, and thus manage to better the quality of clinical data as well as other areas of scientific interest.
One of the most outstanding regulatory agencies requiring studies to be submitted using CDISC standards is Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). This agency is absolutely devoted to the conduct of safety measures and review of applications for marketing approval of medical products and devices in order to improve the country’s public health and safety.
CDISC standards are also required by China’s NMPA, which stands for Center for Drug Evaluation of the National Medical Products Administration. It is responsible for supervising the safety of drugs, medical devices and cosmetics, and regulating their registration.
Ultimately, in accordance with existing FDA guidance, another agency requiring standards for study data submissions, since relatively recently, is the Center for Drug Evaluation and Research (CDER). Together with the Center for Biologics Evaluation and Research (CBER), these agencies strongly encourage the use of study data standards throughout the development phases of medical drugs and products.
How Can a Clinical Trial Sponsor Implement CDISC Standards in its Clinical Trial?
Preparing clinical trial data for regulatory submission to the US FDA is one of the major challenges faced by sponsors nowadays. Indeed, the FDA requires all data to be in SDTM format, a standardization which greatly accelerates the review and approval procedures. Taking this into account, it is highly advisable to implement clinical data collection tools and standards to meet the expectations of the regulatory agency, whether it be the FDA, PMDA, or another institution.
Along the same line of reasoning, another clever idea would be to hire a CRO expert in CDISC. In effect, CDISC expertise and consultancy translate into more efficiency, process improvement, reduced time for regulatory submissions, and better communication among members of clinical research teams.
Admittedly, sponsors continue to play an important role in making decisions on the level of data standardization. However, the extent to which they assist in this endeavor will vary depending on the company’s operational model, budget, and staff.
Be that as it may, the investment into CDISC standardization can positively influence not only clinical data collection and management, but also the way in which CROs and sponsors communicate. In a nutshell, the sooner data collection standardization is applied, the greater the benefits.
What CDISC Services Does Sofpromed Provide?
Sofpromed is a biometrics CRO specialized in providing CDISC services for biotechnology and pharmaceutical companies worldwide. We help clinical trial sponsors collect, analyze, report, and submit clinical trial data according to CDISC standards, by providing a wide spectrum of clinical data management and statistical programming services. Our service portfolio includes the definition and implementation of CDASH-compliant Electronic Data Capture (EDC) systems, and the preparation and submission of clinical data based on the SDTM standard.
If you need CDISC services for clinical trials, please contact us at info@sofpromed.com
References:
[1] Verplancke, Philippe. 2019. “What Is CDISC and Why Does It Matter?” The XClinical Blog. [Accessed 28th January 2022]
[2] Gaddale, Jagadeeswara Rao. 2015. “Clinical Data Acquisition Standards Harmonization Importance and Benefits in Clinical Data Management.” [Accessed 28th January 2022] doi: 10.4103/2229-3485.167101
[3] Formedix. 2020. “All You Need to Know about SDTM.” [Accessed 2nd February 2022]
[4] Altexsoft. 2020. “CDISC Standards: Explaining SDTM, CDASH, ADaM, ODM-XML, and More.” [Accessed 2nd February 2022]
[5] CDISC. “CDISC Standards.” [Accessed 2nd February 2022]