If you need Medical Monitors for clinical trials in the United States, please contact us at info@sofpromed.com Medical Monitors play an important role in clinical trials as they provide the medical...
new articles
How are clinical trials conducted? What is a CRO? Read our articles and learn more about clinical trials and contract research organizations.
Medical Monitors for Clinical Trials in Europe
If you need to hire Medical Monitors for clinical trials in Europe, please contact us at info@sofpromed.com The importance of hiring dedicated Medical Monitors to conduct clinical trials lies in...
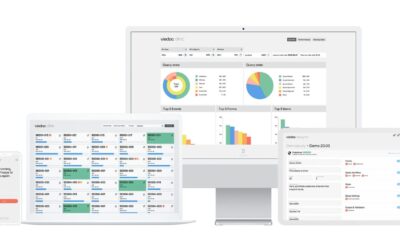
Viedoc: The EDC Solution for Oncology Clinical Trials
For more information about the Viedoc EDC solution, please contact us at info@sofpromed.com Oncology clinical trials are complex and require the use of effective Electronic Data Capture (EDC) tools...
CRO for Phase 1 Oncology Clinical Trials in the United States
If you need a CRO to carry out a phase 1 cancer clinical trial in the United States, please contact us at info@sofpromed.com Are you a biotechnology company planning a phase 1 oncology clinical...
How Much Does a Phase 1 Oncology Clinical Trial Cost in Spain?
If you are looking for a CRO to conduct a phase 1 oncology clinical trial in Spain, please contact us. Spain is a strategic and attractive country to conduct phase 1 clinical trials in oncology. In...
How to Prepare for an FDA Clinical Site Inspection
Contact info@sofpromed.com to learn more about how to prepare for an FDA clinical site inspection You are a hospital or clinic participating in a clinical trial and one day two officers from the...
How to Comply with GDPR in European Clinical Trials
Contact us at info@sofpromed.com if you need support to comply with GDPR in your clinical trial It is important that non-EU clinical trial sponsors meet GDPR principles when conducting their...
CRO for Clinical Trials in France
If you need a Clinical Research Organization (CRO) for clinical trials in France, please contact us at info@sofpromed.com France is one of the most attractive countries to conduct clinical trials...
Expert CRO for Oncology Clinical Trials Worldwide
If you need an oncology CRO to carry out a cancer clinical trial, please contact us at info@sofpromed.com Oncology is one of the main therapeutic areas in which clinical trials are conducted...
The Best CRO for Small Biotech Companies
If you are a small biotech company needing CRO services, you can contact us at info@sofpromed.com Small biotech companies getting started with their first early phase clinical trials have particular...
CRO for Clinical Trials in the United States
If you need a CRO to carry out a clinical trial in the US, please contact us at info@sofpromed.com Are you a biotechnology or pharmaceutical company planning a clinical trial in the United States?...
Exom Group: CRO for Clinical Trials in Italy
Luigi Visani is the President and CEO of Exom Group Srl, an Italian full-service clinical research organization (CRO) based in Milan, and specialized in providing a wide range of clinical trial...
SSS: CRO for Clinical Trials in Germany
Dr. Lars Behrend is the Chief Operating Officer of SSS International Clinical Research, a full-service clinical research organization (CRO) in Germany, specialized in providing clinical trial...
CR Medicon: CRO for Clinical Trials in China
Dr. Henry Wu is the Chief Executive Officer of CR Medicon, a full service clinical CRO in China dedicated to providing high-quality clinical trial management services for the biopharmaceutical...
Hire Clinical Research Coordinators in Spain
Contact us if you need to hire clinical research coordinators (study coordinators) or data entry staff in Spain for clinical trials. Clinical research coordinators (CRC) carry out very valuable and...
Clinical Research Organization (CRO) for Clinical Trials in the United Kingdom
If you need a CRO to manage a clinical trial in the UK, please contact us here Are you a biotech or pharmaceutical company planning a clinical trial in the United Kingdom? Sofpromed is a clinical...
Cost Breakdown for a Phase 1 Clinical Trial in Oncology: A CRO Quote
Click here if you want to download a free clinical trial quotation What is the cost of conducting a phase 1 clinical trial in oncology? Oncology is one of the most common therapeutic areas when it...
The IQVIA, Syneos, ICON, and Parexel CRO Alternative
IQVIA, Syneos Health, ICON, and Parexel are some of the world’s largest global clinical research organizations (CROs). These are full service global CROs with offices spread all around the world and...
Clinical Trial Staff Recruitment for Biotech, Pharma, and Life Science Companies
Click here to download our clinical trial staff price list Are you a biotech or pharma company looking for clinical operations and biometrics staff to work on your clinical trial? Sofpromed...
Synerlab Group CDMO: Drug Manufacturing for Clinical Trials in Europe
Olivier Cadiou is the Business Development Director of Synerlab Group, a European CDMO headquartered in France and specialized in the contract development and manufacturing of pharmaceutical...