If you need SAS statistical programming services for clinical trials, contact us at info@sofpromed.com Statistical programming is an essential element in a clinical trial. In particular, SAS...
new articles
How are clinical trials conducted? What is a CRO? Read our articles and learn more about clinical trials and contract research organizations.
SDTM Services for Clinical Trials
If you need SDTM services for clinical trials, contact us at info@sofpromed.com Ensuring that clinical trial data is SDTM compliant is one of the most significant aspects for a clinical trial that...
Does the FDA Require CDISC?
If you need CDISC services for clinical trials, please contact us at info@sofpromed.com Clinical trial data standardization is important when it comes to data management and clinical data...
How Much Does a Clinical Trial Cost in Europe?
If you need a quote for a clinical trial in Europe, contact us at info@sofpromed.com This is Patricio Ledesma, Head of Clinical Operations at Sofpromed CRO. I would like to explain the details of a...
Guideline for Clinical Trial Application Submissions in the United Kingdom
If you need support for a clinical trial application submission in the UK, please contact us at info@sofpromed.com The United Kingdom is one of the world’s most important countries where clinical...
Quotation for an Electronic Data Capture (EDC) System
If you need a quote for an Electronic Data Capture (EDC) system, you can cotact us at info@sofpromed.com How much does an Electronic Data Capture (EDC) platform cost? This is a question that...
What is a Patient Registry Software?
If you need a patient registry software, please contact us at info@sofpromed.com A patient registry is a clinical database that collects information about patients who are affected by a particular...
Rater Training for Improved Signal Detection in CNS Clinical Trials
If you need rater training services for CNS clinical trials, please contact us at info@sofpromed.com Signal detection poses important challenges in central nervous system (CNS) clinical trials. The...
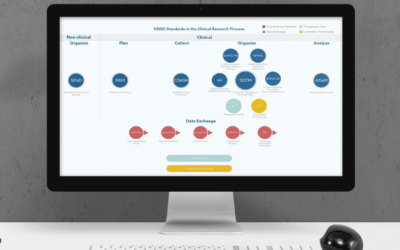
SAS Statistical Programming Services for Clinical Trials
If you need SAS statistical programming services for clinical trials, contact us at info@sofpromed.com Statistical programming is a crucial element in the development of a clinical trial. More...
CDISC SDTM Services for Clinical Trials
If you need CDISC SDTM services for clinical trials, contact us at info@sofpromed.com Biometrics clinical research organizations (CROs) are companies specialized in statistical programming and data...
Oncology CRO for Glioma and Glioblastoma Clinical Trials in the US
Please contact us at info@sofpromed.com if you need a CRO to manage clinical trials in brain tumors in the US The main purpose of conducting clinical trials in brain tumors is to achieve successful...
Clinical Research Coordinators (CRC) for Clinical Trials in the United States
If you need to hire clinical research coordinators (CRC) for clinical trials in the US, please contact us through this link Clinical Research Coordinators (CRCs) play a very important role in...
Spanish CRO for Clinical Trials in Spain
If you are looking for a CRO to conduct a clinical trial in Spain, please contact us through this link Spain is a very strategic country for conducting clinical trials. A large number of clinical...
Sarcoma PDX Models for Drug Testing
If you are interested in sarcoma patient-derived xenograft (PDX) models for drug testing, please contact us at info@sofpromed.com In vivo sarcoma patient-derived xenograft (PDX) models are...
Human Sarcoma Cell Lines for Biotech Companies Testing New Drugs
If you are interested in getting access to patient-derived sarcoma cell lines for drug testing, please contact us at info@sofpromed.com In vitro human-derived sarcoma cell lines are essential tools...
Expert CRO for Leukemia Clinical Trials in the US
Illustrative image of the destruction of a leukemia cell (Dr_Microbe, iStock by Getty Images)Please contact us at info@sofpromed.com if you need a CRO to conduct a leukemia clinical trial in the US ...
Expert CRO for Soft Tissue Sarcoma Clinical Trials in the United States
Please contact us at info@sofpromed.com if you need a CRO to conduct a soft tissue sarcoma clinical trial in the US Are you a small or midsized biotech company planning a clinical trial in soft...
Oncology CRO for Ovarian Cancer Clinical Trials in the United States
If you need a CRO to conduct an ovarian cancer clinical trial in the US, please contact us at info@sofpromed.com Are you a biotechnology or pharmaceutical company looking for a clinical research...
Contract Clinical Research Associates (CRAs) for Cancer Clinical Trials in the United States
If you need contract Clinical Research Associates (CRAs) for oncology clinical trials in the U.S., please contact us at info@sofpromed.com Are you a biotechnology startup company seeking contract...
Clinical Research Associates (CRAs) for Oncology Clinical Trials in the United States
If you need Clinical Research Associates (CRAs) for clinical trials in the U.S., please contact us at info@sofpromed.com Are you a biotech company looking for Clinical Research Associates (CRAs)...