Please click here to request a quotation for your drug manufacturing project
Emerging biotechnology companies planning their early phase clinical trials seek to use contract development and manufacturing organizations (CDMOs) in order to meet their particular fill-and-finish needs.
Contract fill-and-finish services remain one of the most subcontracted activities in the drug manufacturing space today.
New start-up biotech companies will hire the services of a CDMO because they do not have the technical capabilities to manufacture drugs in house.
Sophisticated activities such as lyophilization, filling prefilled syringes, or new technologies require highly specialized manufacturing capabilities that can only be obtained through specialized providers.
Sofpromed, through its partners, provides cGMP high-quality CDMO services for small-batch fill-finish, which is an ideal option for biotech companies conducting their early stage phase I-II clinical studies.
We can provide drug manufacturing services to pharmaceutical and biotechnology companies worldwide.
We are a very agile and competent partner to manage small to medium batches typically used in early phase trials. We can facilitate the required flexibility that bigger CDMOs may not.
We possess wide experience in filling a large number of cGMP finished drug product batches to support clinical trials across multiple therapeutic areas.
We have the skills to handle highly complex formulations, including liposomes, emulsions, nano-particles, small molecules, conjugates, and adjuvants.
In particular, we are experts in aseptic fill-finish services, which we discuss in the following lines.
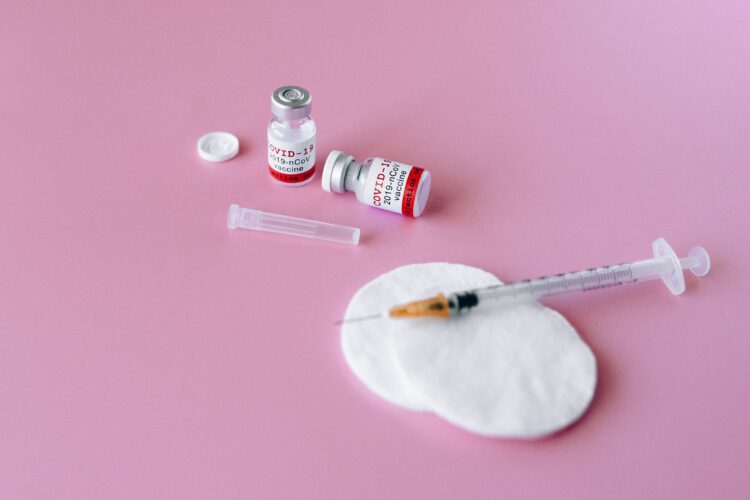
Aseptic Fill & Finish
The fill finish manufacturing market was valued at US$ 6,129. 03 million in 2019 and is projected to reach US$ 12,547. 23 million by 2027; it is expected to grow at a CAGR of 9. 6% from 2020 to 2027 [1].
The growth of the fill finish manufacturing market is mainly attributed to factors such as rising adoption of prefilled syringes for parenteral administration and elevated demand for biologics.
Aseptic filling is an aseptic process that requires the close coordination and complex interaction between personnel, sterilized product, the manual fill/finish equipment system, cleanroom and support facilities, and sterilized filling components.
In an environment where patient safety is imperative, sterility is the most important challenge in fill-finish manufacture.
Any breach in sterility can cause microbial contamination and impact drug purity, placing the batch at risk.
If the contamination is not identified before release, it can harm the patient and cause a product recall.
Since both the risk to the patient and the complexity of the fill-finish process are so high, fill-finish manufacture is subject to extensive inspections, regardless of batch size and production scale.
To ensure sterility, and regulatory compliance, the entire fill-finish process requires highly specialized equipment capabilities and manufacturer support.
The aseptic core in which the sterile drug is actually exposed to the cleanroom environment is the most crucial area of a cleanroom and warrants the most detailed attention to the design of the cleanroom.
This is the area where the sterile drug is transferred from the filling needles to the sterile container.
If the client provides a solution, powder or suspension which has been terminally sterilized, the aseptic fill/finish process can be performed.
Typically, sterile drugs are aseptically filled and finished in molded glass bottles, tubular glass vials, and tubular glass syringes.
We can meet demand for small-scale, shorter sterile drug product development timelines, in compliance with international quality standards.
Do not hesitate to contact us for your next fill-finish project.
Please click here to request a quotation for your drug manufacturing project
For more information about drug manufacturing services, please contact us at info@sofpromed.com
References: