Analytical method development, validation, and transfer are key factors in drug development and manufacturing.
Method development is based on analytical chemistry, which includes methodologies to identify, separate, and quantify the chemical components of medicinal compounds.
This article provides a practical introduction to method development and validation in the context of early phase clinical trials.
What is analytical method development?
The purpose of analytical method development is to establish the identity, purity, physical characteristics, and potency of drugs, including the drug’s bioavailability and stability.
Analytical method development and validation can be understood as the process of showing that analytical procedures are adequate for the purpose of assessing drugs, and particularly the active pharmaceutical ingredient (API).
Analytical procedures are developed to test specific characteristics of the substances against the predefined acceptance criteria for such characteristics.
Thus, analytical method development involves the evaluation and selection of the most precise assay procedures to determine the composition of a drug.
Why is analytical method development important for biotech companies conducting early phase clinical trials?
Analytical method development and validation is tremendously important for any drug development program.
We can identify at least three main reasons why analytical method development is critical for any biotechnology company developing new drug candidates.
Firstly, the quality of a drug is obviously at the core of the success possibilities of a pharmaceutical development program, so that biotech companies developing innovative compounds must take analytical method development very seriously.
Secondly, analytical method validation is required by regulatory authorities worldwide for both clinical trial applications and marketing authorizations.
For example, a biotech company having an Investigational Medicinal Product Dossier (IMPD) for its drug, with a low-quality chemistry, manufacturing, and controls (CMC) section will have a difficult time to have its clinical trials approved by European drug agencies. Then, method development is key to have clinical studies approved.
Finally, after all, patients will be the ones eventually receiving the investigational medicinal product (IMP) in early phase clinical trials (first in human / Phase 1 studies), so the development and manufacturing quality of a medicine is vital to ensure patient safety and hopefully see promising efficacy in the new treatments.
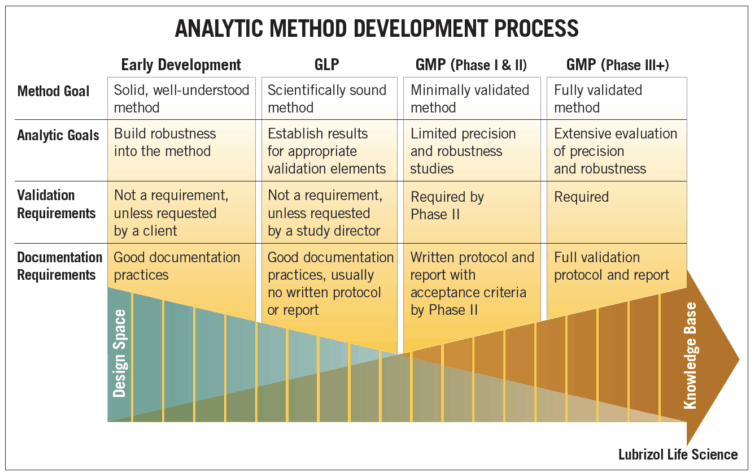
The figure below provides an overview of the analytic method development process, including method goals, analytic goals, validation requirements, and documentation requirements at the different stages of drug development.
What are the most common types of analytical procedures?
The most common types of analytical procedures include identification tests, quantitative tests for impurity content, limit tests for impurity control, and quantitative tests for the active moiety in drug substance or drug product.
What are the main parameters involved in drug analytical testing?
When talking about analytical methods in drug development, qualitative and quantitative methods should be differentiated.
Methods for compound testing include validation parameters such as specificity, limit of detection, limit of quantitation, linearity, accuracy, range, precision (under laboratory repeatability conditions), and stability.
In addition, revalidation may be required if changes are introduced in the synthesis of the drug substance, in the composition of the drug product, or if modifications are made to the analytical procedure.
What are the main steps involved in drug method development?
Dr. Joanna Greenhough has written a very useful article on pharmaceutical method development and validation, in which the following is stated regarding the lifecycle of an analytical method:
“The lifecycle of an analytical method starts when a pharmaceutical company or a contract analytical testing laboratory recognises a requirement for a new analytical method.
They will then either identify an existing/compendial procedure suitable for the particular need or proceed to develop a completely new method.
Following the development stage, a validation (or verification) plan will be prepared and the method validation experiments will be performed.
The process of validation should follow a validation protocol which must clearly define the application purpose and scope of the method, performance characteristics with acceptance criteria, validation experiments, standards and reagents.
Once the analytical method validation confirms the method’s suitability for its intended purpose, the standard operating procedures (SOPs) for the routine execution of the method need to be developed and approved.
As the analytical method should be continually monitored for its fitness for purpose throughout its lifecycle, the criteria for revalidation and type/frequency of system suitability tests and QC checks should be defined.
Following the successful submission of the validation report, the analytical procedure can be used for routine analysis.
If changes to the method occur, the evaluation of their effect on the procedure’s suitability for its intended use is essential.
If changes applied to the analytical method are covered by current validation, no further validation is necessary.
Otherwise, any changes falling beyond the scope of existing validation will result in either revalidation or, sometimes, method redevelopment and new validation.”
What is analytical method transfer?
According to the general US Pharmacopeia (USP) chapter 1224:
“The transfer of analytical procedures (TAP), also referred to as method transfer, is the documented process that qualifies a laboratory (the receiving unit) to use an analytical test procedure that originated in another laboratory (the transferring unit), thus ensuring that the receiving unit has the procedural knowledge and ability to perform the transferred analytical procedure as intended.”
Analytical method transfer is typically managed under a transfer protocol that details the parameters to be evaluated in addition to the predetermined acceptance criteria that will be applied to the results.
Transfer studies usually involve two or more laboratories (originating lab and receiving labs) executing the pre-approved transfer protocol.
What regulatory guidelines must be considered when preparing method development data in early phase clinical trials?
Pharmaceutical method development and validation work must be done according to established international guidelines such as those published by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), the US Food and Drug Administration (FDA), and the European Medicines Agency (EMA).
The FDA and US Pharmacopoeia (USP) both refer to ICH guidelines.
Of course, analytical methods must be validated following good manufacturing practice (GMP) and good laboratory practice (GLP) directives.
Here you can see relevant guidance documents:
- Validation of Analytical Procedures: Text and Methodology Q2(R1), ICH 2005
- Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics, FDA 2015
- <1225> Validation of Compendial Procedures, USP 39
- <1226> Verification of Compendial Procedures
- <1058> Analytical Instrument Qualification
- EMA Guideline on the requirements for the chemical and pharmaceutical quality documentation concerning investigational medicinal products in clinical trials
If you are planning a clinical trial in Europe, you can benefit from reading our article entitled “25 Tips for the IMPD Quality Section: Guidance for Clinical Trials in Europe”, which includes observations related to analytical method development.
What kind of companies can help me with analytical method development?
There are companies specialized in providing analytical method development and validation services for new drugs.
These types of companies are commonly known as contract development and manufacturing organizations (CDMOs).
Sofpromed can facilitate these services through laboratories located in the United States and Europe.
Conclusion
Analytical method development, validation, and transfer are crucial aspects in drug development.
Method development is critical for a biotech company for commercial, regulatory, and patient safety reasons.
Without high quality method development and validation in place, it is impossible to have clinical trials approved or marketing authorizations granted.
Counting on the support of a competent CDMO ensures success in a drug development program, at least as far as CMC aspects are concerned.
Please contact us at info@sofpromed.com if you need further information.